Outline
Recently, Prof. Zhenning Liu’s group at the Key Laboratory of Bionic Engineering of Jilin University has published an article entitled "Spermidine-functionalized biomaterials to modulate implant-induced immune response and enhance wound healing" on Chemical Engineering Journal. Prof. Liu’s group has prepared two types of spermidine-functionalized implantable biomaterials with a crosslinker consisting of spermidine (SPD) and terephthalaldehyde (TA): one is hydrogels (SPD-DN) from natural biopolymers (e.g. chitosan from shrimp shell and gelatin from porcine skin) and the other is composite films (SPS) based on synthetic polymers (e.g. polypropylene carbonate). Both spermidine-functionalized hydrogels and films show good mechanical properties, swelling properties, cytocompatibilities and pro-healing properties. More importantly, these two spermidine-functionalized biomaterials demonstrate low immunogenicity, which is manifested by reduced pro-inflammatory cytokines in rats and accelerated transition from M1 macrophage-dominated phase to M2 macrophage-dominated phase. Hence SPD-functionalized biomaterials can inhibit implant-induced foreign body reaction (FBR) and direct a more natural wound healing. This work has afforded a nature-inspired approach to mitigate FBR with SPD, which may be a game changer for medical implants and even organ transplantation. The corresponding author of the article, Prof. Liu, is a Ph.D. from Harvard Medical School. Ph.D. candidate Li Wang and Ph.D. Yinghui Zhong are co-first authors.

Background
Medical implants often induce foreign body reaction (FBR) from the host immune system, which is usually featured by aseptic inflammation and fibrous encapsulation around undegradable implants. In clinics, immunosuppressants are used to regulate immune system and mitigate FBR. Unfortunately, these immunoregulatory agents can cause undesirable side effects.
During the fertilization of mammals, male sperms need to enter the female uterus to fertilize the egg. However, sperms are foreign to a woman’s body and the female immune system will be triggered to kill these “invaders”. Spermidine (SPD), as suggested by the name, is a biogenic polyamine originally identified in semen and later found to exist widely in the human body with important functions. It is known that the level of SPD is significantly lower in the semen of infertile men [1,2]. Hence, it has been speculated that SPD can modulate immune responses to protect sperms, which are regarded as “foreign bodies” by the female immune system, to facilitate fertilization, a critical step in natural selection.
Key Points
Point 1: The SPD crosslinker was prepared by the Schiff base reaction between the amino groups of SPD and the aldehyde groups of terephthalaldehyde (TA), an aromatic dialdehyde. The imine bonds formed between SPD and TA are conjugated to the benzyl ring and thus more stable than aliphatic imine bonds. Such an approach can achieve the slow release of SPD, leveraging the reversibility of Schiff base reaction and avoiding burst release and fast metabolic consumption of SPD. It should also be noted the molar ratio of SPD:TA is 1.0:1.2 in the crosslinker so that the crosslinker contains an excess of aldehyde groups to crosslink the amino groups on the biopolymers used to prepare SPD-functionalized hydrogels (SPD-DN) and composite films (SPS) in this work.
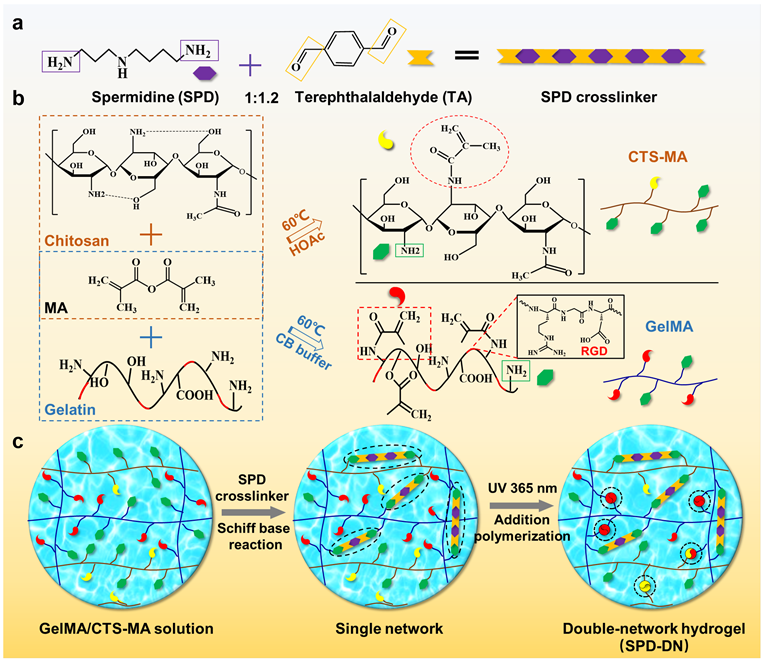
Fig. 1. Schematic illustrations for the preparation of SPD-functionalized hydrogels
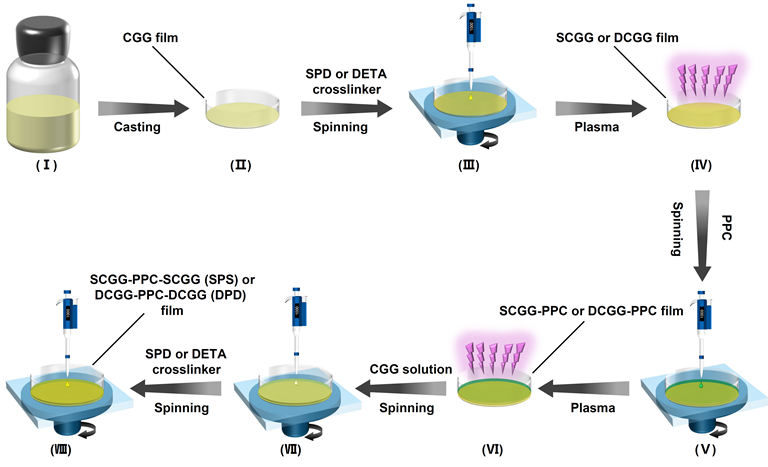
Fig. 2. Schematic illustrations for the fabrication of SPD-functionalized composite films
Point 2: In fact, both exogenous biopolymers and synthetic polymers are recognized as “foreign bodies” by hosts and can trigger immune system to exhibit aseptic inflammation related to FBR. In order to confirm the biological effect of SPD, diethylenetriamine (DETA), a sham molecule with chemical structure similar to SPD, was used in place of SPD to prepare the control crosslinker and respective hydrogels (DETA-DN) and composite films (DPD). Compared to DETA, SPD has two more methylene groups on one side of the secondary amine and one more methylene group on the other side. Therefore, DETA-crosslinked biomaterials are comparable to SPD-functionalized biomaterials in terms of physical and chemical properties. Yet, their biological activities are quite different, which enables an “operando” analysis on the function of SPD. The two SPD-functionalized biomaterials can promote the polarization of RAW 264.7 macrophages from M1-type to M2-type, inhibit the lipopolysaccharide (LPS)-stimulated expression of pro-inflammatory factors, such as interleukin 6 (IL-6), tumor necrosis factor a (TNF-a) and nitric oxide (NO), and enhance the production of anti-inflammatory cytokine interleukin 10 (IL-10). Conversely, no such effects have been found for DETA-crosslinked biomaterials.
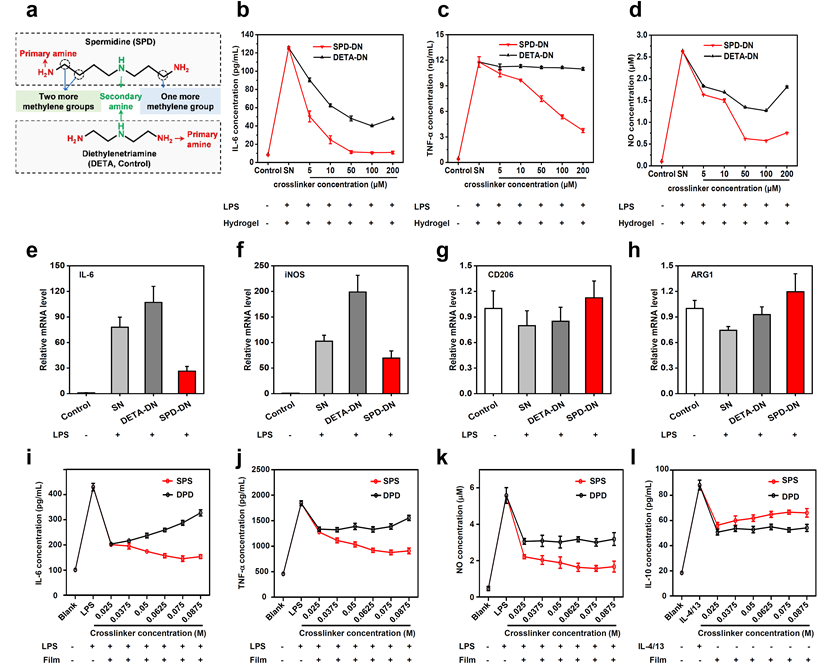
Fig. 3. In vitro effects of SPD-functionalized biomaterials on cytokines and NO
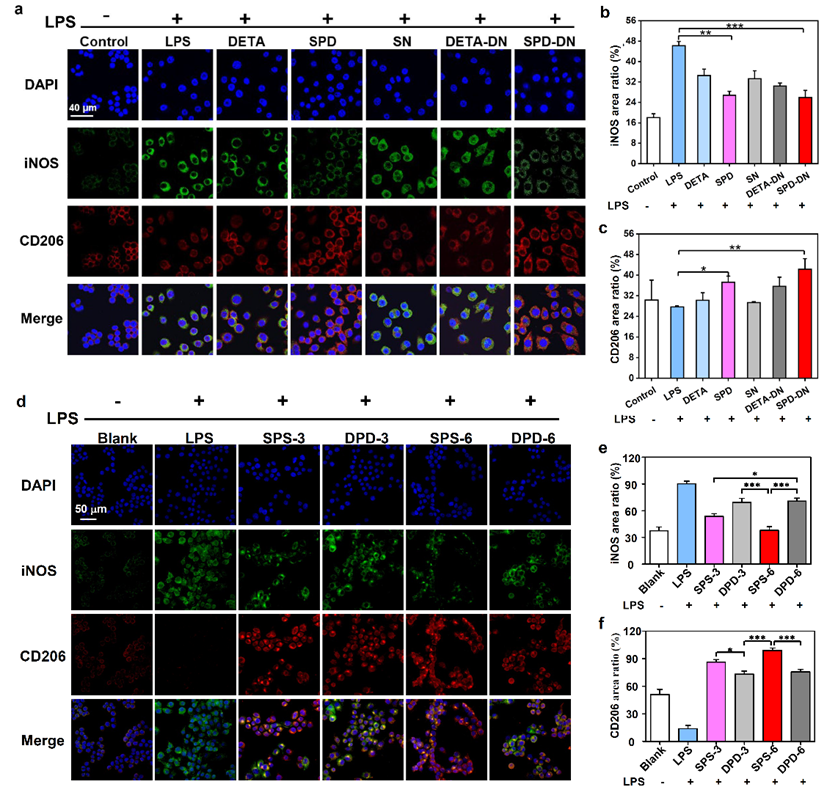
Fig. 4. In vitro regulation of macrophage polarization by free SPD and SPD-functionalized biomaterials
Point 3: SPD-functionalized hydrogels (SPD-DN) show minimal immunogenicity after implantation in rats. More intriguingly, the inflammatory response against SPD-DN hydrogels attenuates as the SPD concentration increases. In particular, the infiltration of immune cells for SPD-DN hydrogels at a crosslinker concentration of 500 micromolar is comparable to that of the sham control without implantation. In contrast, the surrounding tissues of DETA-crosslinked hydrogels exhibit massive immune cell infiltration, suggesting severe inflammation and FBR against exogenous objects. In addition, both SPD-functionalized hydrogels and composite films can downregulate pro-inflammatory cytokines and accelerate the transition from M1-dominated phase to M2-dominated phase, which leads to better collagen deposition and angiogenesis as well as faster wound healing. As a result, the skin regenerated with SPD-functionalized biomaterials better resembles native skin.
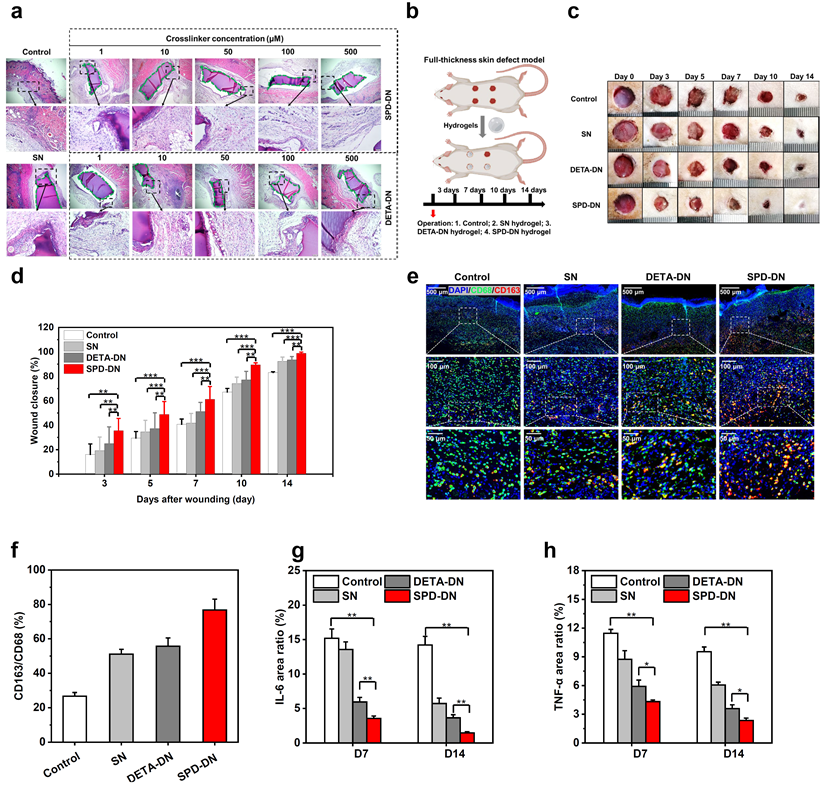
Fig. 5. In vivo immunomodulation and wound healing by SPD-functionalized hydrogels (SPD-DN)
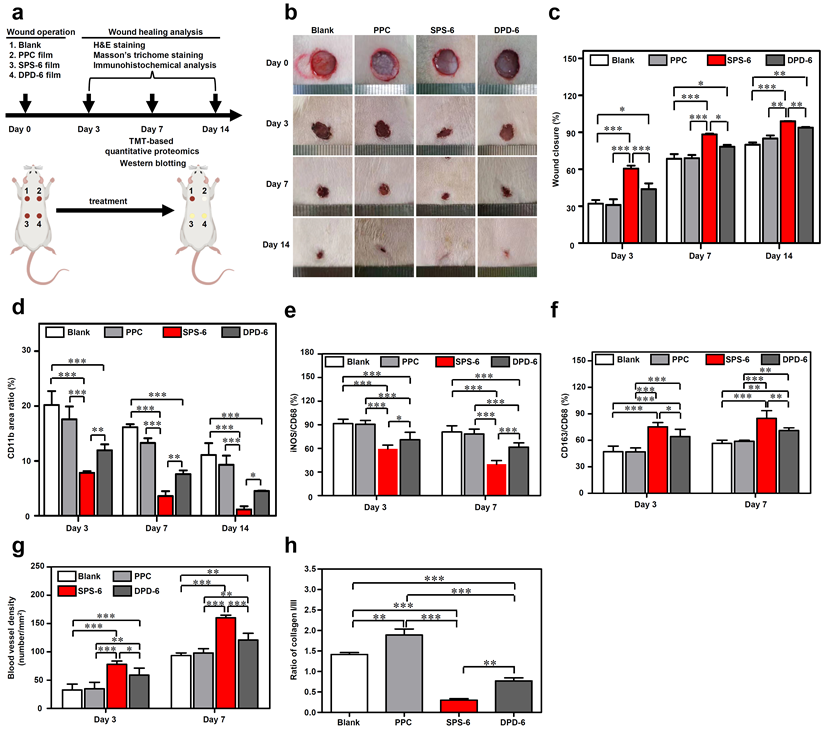
Fig. 6. In vivo immunomodulation and wound healing by SPD-functionalized composite films (SPS)
Point 4: Proteomic analysis by tandem mass tags (TMT) has revealed possible immunoregulatory mechanisms for SPD-functionalized biomaterials. SPD-functionalized biomaterials can not only inhibit inflammatory response mediated by NF-κB signaling, but also activate mitochondrial function via AMPK pathway, thus promoting tricarboxylic acid (TCA) cycle and β-oxidation of fatty acids. Hence, it is found that SPD can accelerate the polarization of macrophages toward M2 phenotype, mitigate implant-induced inflammation and enhance wound healing by the synergy between reducing pro-inflammatory factors and metabolic reprogramming.
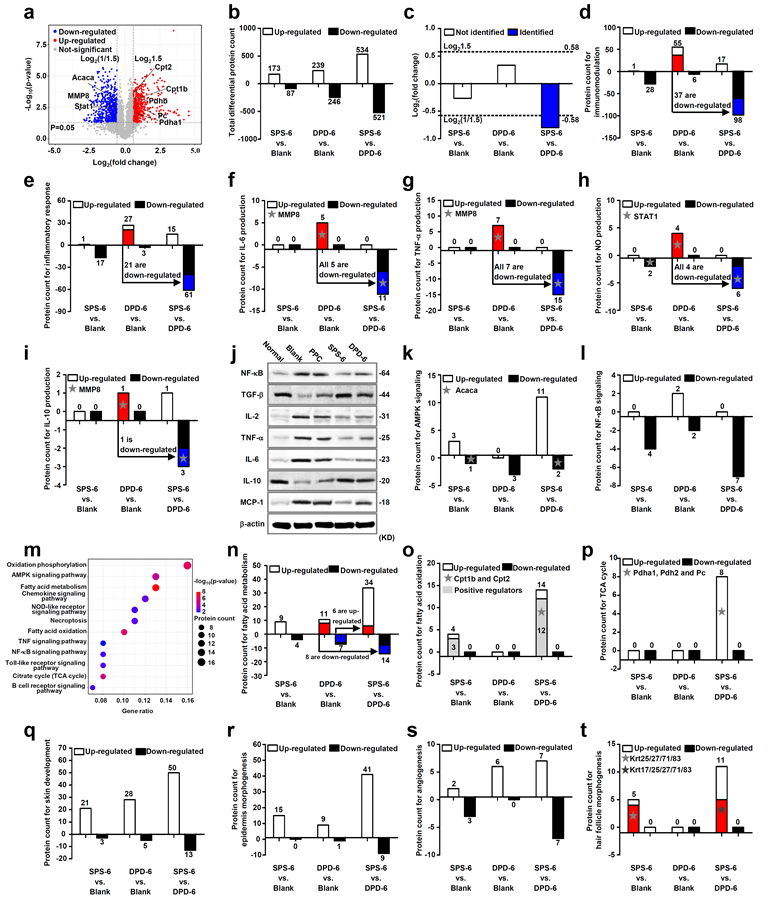
Fig. 7. In vivo proteomic analysis for SPD-functionalized composite films (SPS)
In summary, the local administration of SPD in implantable biomaterials can reduce implant-induced aseptic inflammation and FBR, as manifested by the down-regulation of pro-inflammatory cytokines at the early stage of wound healing and faster transition from M1 macrophage-dominated phase to M2 macrophage-dominated phase. Such an immunomodulatory effect by SPD leads to enhanced healing results that better resemble native skin. Hence, the use of SPD in medical implants may afford a simple but effective approach to change the fate of implanted medical devices for a better outcome.
This work was supported by the National Key R&D Program of China (2022YFE0138500), National Natural Science Foundation of China (51975245 and 21975097), Jilin Provincial Science & Technology Department (20200404166YY) and Program of Jilin University Science and Technology Innovative Research Team (2020TD-03).
References:
[1] P.L.C. Lefevre, M.F. Palin, B.D. Murphy, Polyamines on the Reproductive Landscape, Endocrine Reviews 32(5) (2011) 694-712. https://doi.org/10.1210/er.2011-0012
[2] E. Proietti, S. Rossini, U. Grohmann, G. Mondanelli, Polyamines and Kynurenines at the Intersection of Immune Modulation, Trends in Immunology 41(11) (2020) 1037-1050. https://doi.org/https://doi.org/10.1016/j.it.2020.09.007
[3] Li Wang, Yinghui Zhong, Qianqian Wu, Yu Wang, Ruoqi Tang, Silu Zhou, Jingde Yang, Qiming Liu, Guoxin Shi, Yanan Tang, Xianglong Meng, Kexin Chen, Xianqiang Yan, Xuelei Liu, Jing Zhan, Thomas M. Roberts, Song Liang, Jiaao Yu, Zhenning Liu, Spermidine-functionalized Biomaterials to Modulate Implant-induced Immune Response and Enhance Wound Healing, Chemical Engineering Chemical 476(2023). https://doi.org/10.1016/j.cej.2023.146416
Copyright © 2024 International Society of Bionic Engineering All Rights Reserved
吉ICP备11002416号-1









